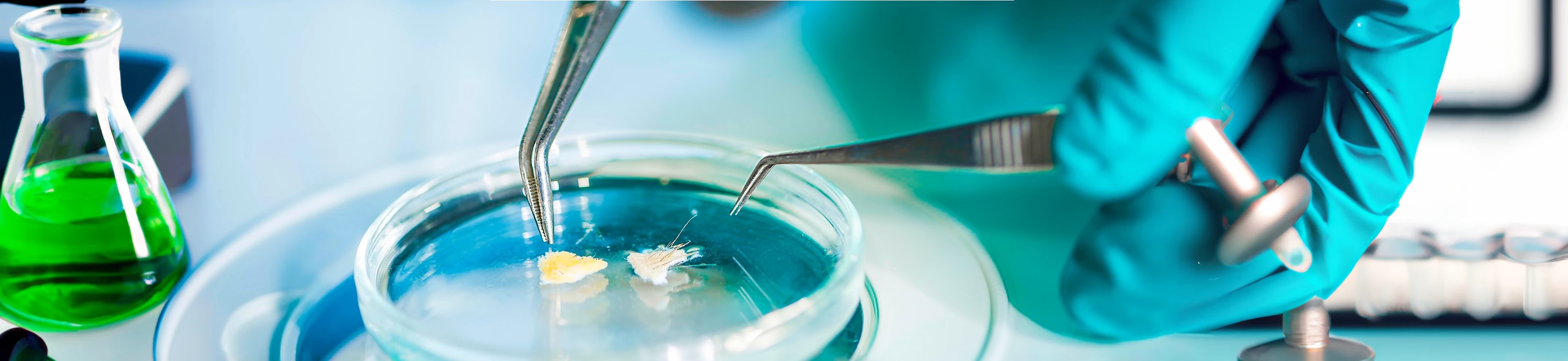
[Inno 360°] Minimal ingredients, maximal tolerance 🤩
➡️ Discover the sterile cosmetics, a Pierre Fabre Laboratories innovation 👏
Discover
Our Brands
Discover
Our Brands
Our dual expertise - pharmaceutical and dermo-cosmetics - helps us offer a holistic approach to care: prevent, treat and support.



Focus on
Our Commitments
Focus on
Our Commitments
Green Mission Pierre Fabre is the eco-socio-responsible commitment from the Pierre Fabre Group, awarded the Committed to CSR by AFNOR Certification at the Exemplary level. Thanks to our conviction and drive, we use sustainable innovation to benefit Nature and People, focusing on 5 pillars
Innovate
Protect
Respect
Guarantee
Engage

Looking for a company
that is in line with your values?
Looking for a company
that is in line with your values?
Joining the Pierre Fabre Group means entering a company with a history overflowing with values, innovation and expertise for patients and consumers. Don't wait! Look at our offers, apply and embark on a human adventure !
Our latest
News
Our latest
News
Curious to learn more about our news? Get the latest on our brand innovations, our flagship commitments and the highlights of the life of the Group