Recent updates in BRAFV600E-mutant metastatic colorectal cancer treatment
1 February 2023
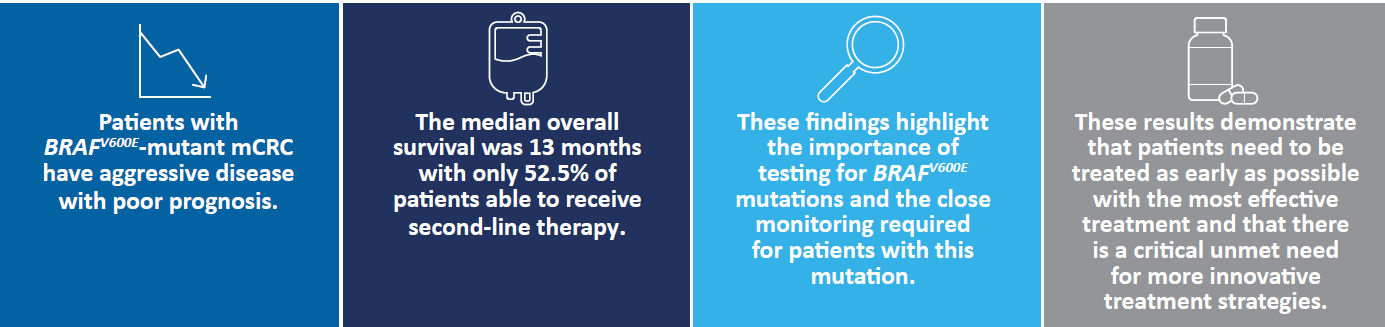
In 2020, almost two million people were diagnosed with colorectal cancer (CRC)1. At present, survival rates for the metastatic CRC (mCRC) population are poor, with less than 20% of people living for more than five years after diagnosis across Europe. Of these patients, 8-12% are diagnosed with a BRAF mutation, 95% of which are BRAFV600E, an aggressive form associated with a poor prognosis.2

Sadly, BRAFV600E-mutant mCRC is a distinct and particularly aggressive form of mCRC and survival rates are poor compared to other sub-types. Historically, as patients with this mutation usually make up a small sub-group of larger mCRC studies, there has been limited data available from which to draw firm conclusions regarding best treatment practices. Therefore, evaluation of real-world treatment practices, especially in the first-line setting, is important in order to characterize this patient group better.
The CAPSTAN study, which is the largest real-world study of its kind, has very recently been published in ESMO OPEN and described the treatment patterns of BRAFV600E-mutant mCRC in a real-world setting across Europe.2 This is the first retrospective study to assess the treatment of BRAFV600E-mutant mCRC in routine clinical practice across Europe between 2016-2020, with the aim of defining initial testing and treatment patterns, characterising the disease and treatment effectiveness, and safety.2
Watch this short video of Professor Dirk Arnold ( Head of Oncology, Haematology & Palliative care, AK Altona, Hamburg and Director, Aklepios Tumorzentrum, Hamburg) to find out his perspectives on what the CAPSTAN study has shown and why these findings are important for patients diagnosed with BRAFV600E-mutation mCRC.
Data from this study clearly shows that patients with a BRAFV600E-mutation are presenting with more advanced and aggressive disease compared to CRC overall.2
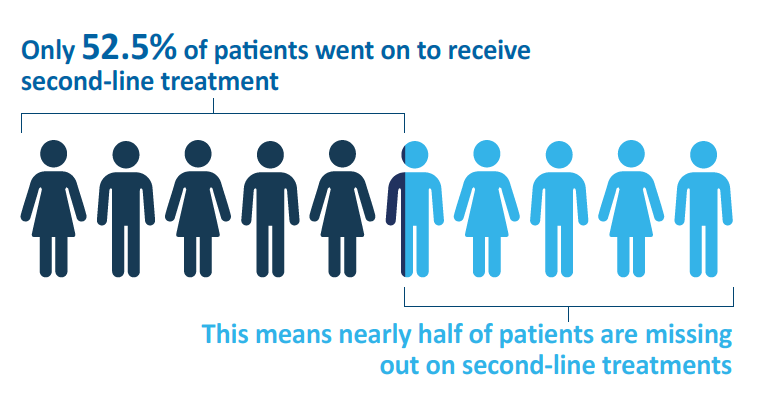
Only 52.5% of patients were able to receive a second line of treatment, highlighting the need for close monitoring and more effective treatment options. This may improve the outcomes for patients with BRAFV600E-mutant mCRC, and allow more patients to receive further treatment.2
In addition, the study’s findings highlight the importance of testing for BRAFV600E-mutations to personalize and optimize treatment.2

|
The newly published Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up also recommends testing of BRAF mutations in all patients when mCRC is first diagnosed, as this can influence the initial treatment they receive3. |
While the treatment options are limited for patients with this aggressive and unique type of mCRC, the publication of the CAPSTAN study and the updates to the ESMO guidelines demonstrate a steadfast commitment by the CRC community to generate data and recommendations to help understand and improve treatment practices for this underserved patient population in the future.
Reference:
1. The Global Cancer Observatory, 2020. International Agency for Research on Cancer, World Health Organization. Available at: https://gco.iarc.fr/today/online-analysis-multi-bars. Accessed December 2022.
2. Martinelli E, Cremolini C, Mazard T, Vidal J, Virchow I, Tougeron D, Cuyle PJ, Chibaudel B, Kim S, Ghanem I, Asselain B, Castagné C, Zkik A, Khan S, Arnold D. Real-world first-line treatment of patients with BRAFV600E-mutant metastatic colorectal cancer: the CAPSTAN CRC study. ESMO Open. 2022;7(6):100603.
3. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E, on behalf of the ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology. 2022. doi: https://doi.org/10.1016/j.annonc.2022.10.003.
HQ-11-22-2200032

