At the heart of our targeted oncology research
At the heart of our targeted oncology research
Unique to France, the Toulouse Oncopole is a medical and scientific campus that brings together researchers, health workers, manufacturers and startups, working to fight cancer. It is where Pierre Fabre Laboratories elected to base its Innovation Center in 2010, and, more specifically, its oncology research and development teams. These teams are 100% focused on developing new therapeutic options, from discovery to clinical trials, through translational research.
Identifying candidates for clinical development.
The very first step in any R&D project is to identify and vali- date therapeutic targets. Diseases arise when one or more bodily functions are impaired. In order to develop a new drug, we need first and foremost to understand what is preventing the body from functioning properly.
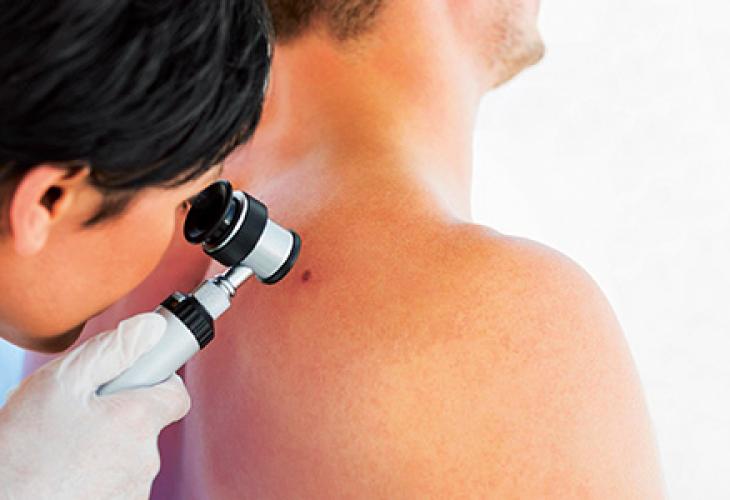
In oncology, the therapeutic target might be an abnormal protein linked to a genetic cell mutation, for instance.
Once we have determined the target, the next step involves finding a molecule that will interact with the target to eliminate or inhibit it. Hundreds of thousands of molecules are tested in vitro in the laboratory or in silico in computerized simulation models. Compounds that are identified as positive are then optimized to improve their pharmacological proper- ties, leading to the final selection of a candidate for clinical development.
To ensure all those steps run smoothly, a team of around 60 biologists, pharmacologists and medicinal chemistry experts work together in research laboratories. Olivier Geneste, Head of Discovery, explains: “We use advanced tech- nologies, such as genetically-modified cancer cells and genomic-based approaches, to decode the way potential therapeutic targets operate.”
The researcher continues: "The partnership we built in 2023 with Vernalis gave us access to innovative screening methods for identifying and refining the molecules that manage to modulate the chosen target. Finally, we characterize the selected molecules for their therapeutic potential in preclinical disease models, along with their pharmacological properties and safety."
Throughout this process, which lasts for 4 to 6 years, other functions provide essential knowledge, such as data science, pharmacokinetics, toxicology, pharmaceutical development and translational research.
Translational research: determining the biomarkers for cancerous tumors
Translational research bridges the gap between biomedical research (in laboratories) and clinical research (in hospitals). The purpose of this cross-functional discipline is to convert scientific discoveries into concrete treatments for patients. Its experts identify patient groups that are likely to respond effectively to the new treatment. To do so, they analyze the genomic and molecular features of the targeted tumor in those patients. They match specific biological markers (biomarkers) with each type of tumor.
A Pierre Fabre Laboratories internal “bio- base” has been created using tumor biopsies covering a broad range of cancer types and their related oncogenes. Using this collection of tumor tissues, researchers and their teams identify and validate the biomarkers that help choose which patients will be enrolled in the clinical trial of the new treatment.
Catherine Regnier, Head of Translational Research, is thrilled about the benefits: “By developing pioneering therapies targeting different onco- genes combined with specific biomarkers, we are able to offer increasingly personalized medical treatments to care for patients more and more effectively.”
Her team is currently characterizing a set of biomarkers to back up the clinical trials of a new monoclonal antibody (known as PFL-002/ VERT-002) whose mechanism of action inhibits the c-MET target. In its mutant form, this target is found in patients suffering from a very specific type of lung cancer.
Clinical development: assessing the tolerance profile, safety and efficacy of new therapies
Clinical development is the last phase in the innovation process, and involves three sequential phases. The goal of phase I is to assess the safety of the clinical candidate and define a recommended dosage and administration plan for patients. Phase II aims to confirm the efficacy of the drug candidate among a specific patient population. Phase III is designed to assess the long-term efficacy and benefits of the new drug among a larger cohort of patients, against the best therapy currently avail- able. If the results are positive, a marketing authorization application can be submitted to the health authorities.
Before each phase begins, a protocol has to be drawn up for the start of the clinical trial. It lays down the patient enrollment criteria along with the methodology, assessment criteria and phases of the trial. Claire Fabre, Head of Clinical Development, stresses: “Here at Pierre Fabre, we aim for clinical excellence, applying the high- est ethical standards. We work closely with health authorities and the hospitals where the clinical trials are conducted, to guarantee the quality of our trials worldwide. We aim for agile opera- tional implementation, so that our oncology trials can be deployed as quickly as possible, based on a clear, innovative scientific rationale.”
Leveraging data and Al to power R&D
Pierre Fabre capitalizes on advanced techno- logies to take its research to the next level. Thanks to data science, large volumes of data about molecules and genomes, taken from public and private databases, are analyzed and used by Pierre Fabre researchers. These analyses are used, among other things, to select therapeutic targets and identify biomarkers, to more quickly develop the right treatments for the right patients.
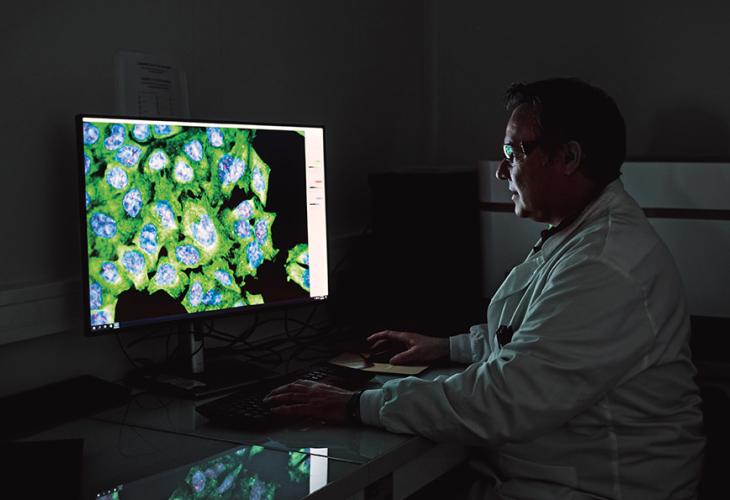
In 2023, Pierre Fabre teams developed proprietary AI for the characterization and sorting of cancer cells. This AI allows us to correctly identify clonal populations, predict resistance mechanisms and separate the tumor environment from the tumor itself, all with the aim of improving the efficacy of our targeted therapies.