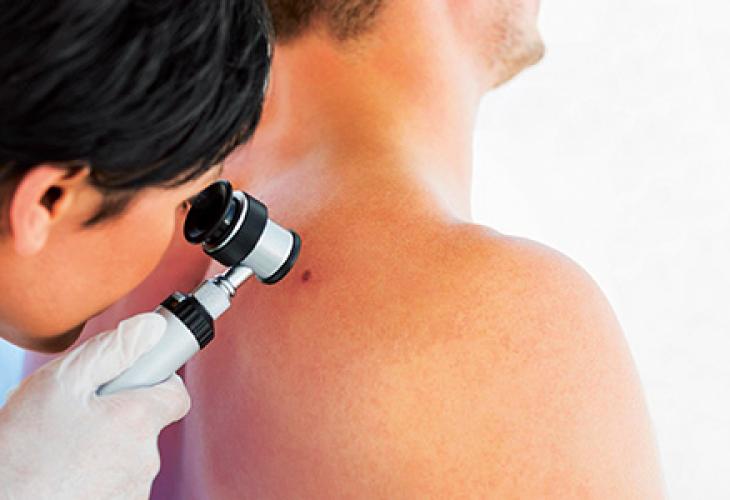
Photo report : Inside our Innovation Center at the Oncopole.
Photo report : Inside our Innovation Center at the Oncopole.
Pierre Fabre Laboratories is a pioneer in the field of oncology and has made the fight against cancer an absolute priority. The teams dedicated to research and development in oncology pursue their objectives at the heart of the Toulouse Oncopole, a medical and scientific campus that is unique in mainland France.

On the Pierre Fabre premises at the heart of the Oncopole, a team of around sixty biologists, pharmacologists and experts in medicinal chemistry work closely together to drive research forward.

While working on their missions, Pierre Fabre’s teams aim for clinical excellence, applying the highest ethical standards.

Throughout the entire research process, the teams based at the Oncopole work in close collaboration with numerous experts in other specialties: pharmacokinetics, toxicology, pharmaceutical development, etc.

Every day, the teams apply advanced technologies, such as genetically-modified cancer cells that are used to decode the way therapeutic targets operate.

Identifying and validating therapeutic targets constitute the first step in the projects conducted by the Pierre Fabre teams at the Oncopole. The objective is to understand what is preventing the body from functioning properly before developing a drug.
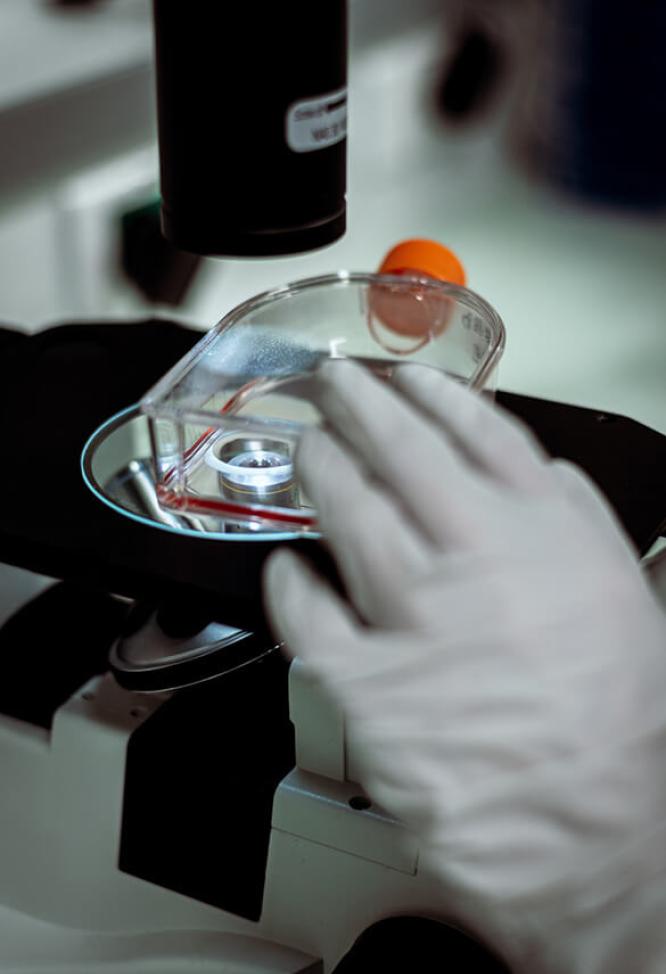
Once the therapeutic target has been determined, the R&D teams work on finding the molecule that will interact with that target in order to eliminate or inhibit it.

Within the Oncopole, Olivier Geneste, Head of Discovery (left), and his teams develop strategic partnerships with numerous biotechs to advance their research.
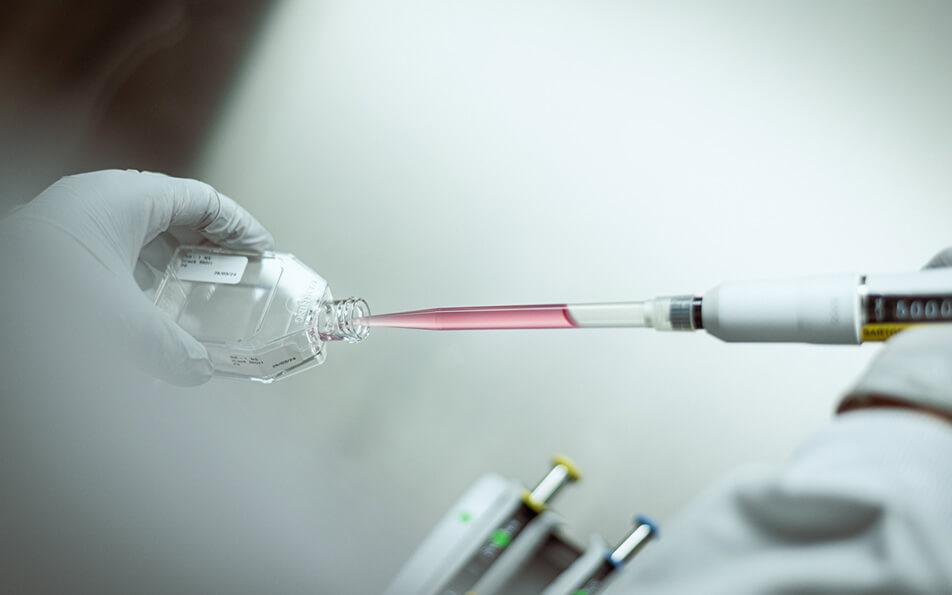
The processes involved in the research into therapeutic targets and the molecules that will interact with them can last between 4 and 6 years.

The Pierre Fabre teams have created an internal “bio-base”: a collection of tumor tissues enabling the researchers to validate the biomarkers that will be used to select patients eligible for the clinical trial.
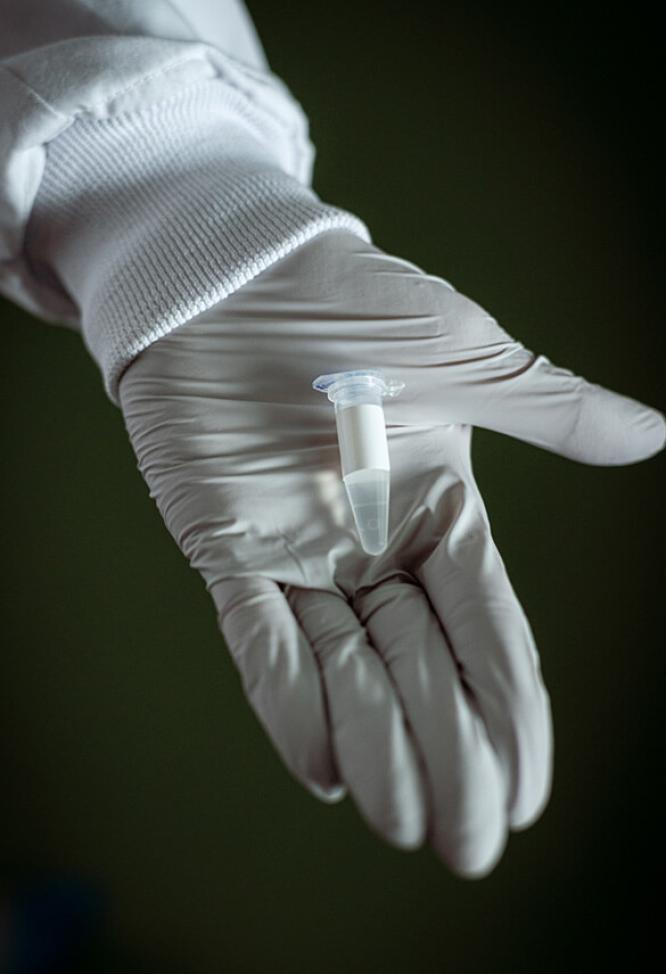

Clinical development is the last phase in the innovation process carried out by the researchers at the Oncopole.

Throughout the clinical development phase, the researchers adhere to a strict protocol defining the patient inclusion criteria, methodology, assessment criteria and the steps in the study.